Phencyclidine Drug Information
Classification
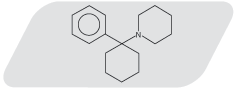 Phencyclidine (l- phencyclohexyl piperidine, PCP) was first introduced in 1956, for use as an intravenous anesthetic. Pharmacologically PCP is classified as a dissociative anesthetic. PCP is currently a popular drug of abuse and was once used as a veterinary tranquilizer. PCP is self-administered either by smoking (drug-laced tobacco, marijuana, or parsley), by nasal insufflation, intravenous injection, or by oral ingestion.
Phencyclidine (l- phencyclohexyl piperidine, PCP) was first introduced in 1956, for use as an intravenous anesthetic. Pharmacologically PCP is classified as a dissociative anesthetic. PCP is currently a popular drug of abuse and was once used as a veterinary tranquilizer. PCP is self-administered either by smoking (drug-laced tobacco, marijuana, or parsley), by nasal insufflation, intravenous injection, or by oral ingestion.
Metabolism
PCP is a lipophilic drug with a large volume of distribution. PCP is stored in adipose and brain tissue with cerebrospinal fluid concentrations being 6-9 times greater than serum concentrations. PCP undergoes extensive hepatic oxidative metabolism with about 10-15% of a dose excreted unchanged in urine. Renal excretion of PCP is decreased when urine is alkaline. Frequent or chronic PCP users may excrete PCP for 2-10+ days following last use.
Abuse
Phencyclidine's pharmacological actions are complex but most are mediated by its antagonism of the N-methyl-D-aspartate receptors. The relationship between dose and clinical effects is not predictable. PCP has stimulant, depressant, hallucinogenic, and analgesic properties. Adverse effects are unpredictable and include agitation, delusions of grandeur, anxiety, hostility, stupor, paranoia, and coma. Death has been known to result following the ingestion of 120 mg of PCP (toxic dose 10-20 mg).
Methods of Analysis
The immunoassay methods (EIA) are widely used screening methods designed to specifically detect phencyclidine and its inactive metabolites. Commonly used confirmation methods include gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). These methods offer excellent sensitivity and specificity and are the methods of choice for most applications. False positive immunoassays have been reported following the use of thioridazine (Mellaril), chlorpromazine (Thorazine), dextromethorphan, or diphenhydramine (Benadryl), therefore indicating the necessity for specific secondary confirmation testing.
800-255-2159
RTL also offers on-site test devices. Click here to view the complete line of Reditest® screening devices.
Drug information data is not definitive and should be used for reference guidelines only.